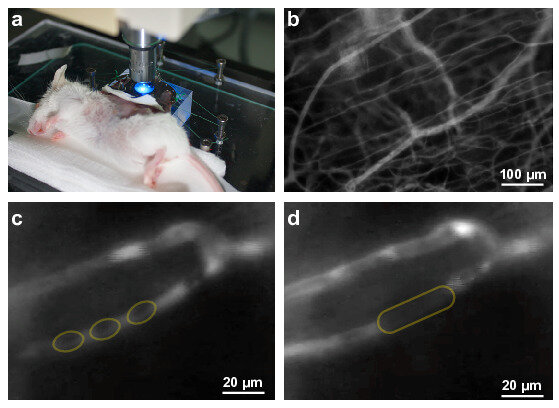
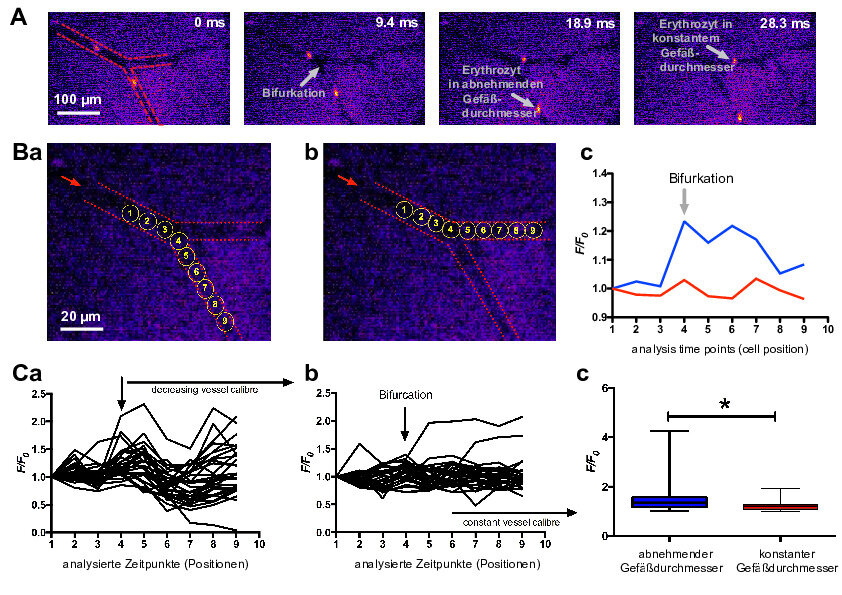
The focus of the research is on the maturation and adaptation of red blood cells (RBC).
After exiting the bone marrow, reticulocytes mature to form the highly adapted red blood cells travel through our circulation during their entire lifetime of in average 120 days. Thus, they are in constant move and adapt to their surrounding by shape changes, be it during in high-speed flow or during severe volume adaptations when they squeeze through small capillaries or the slits of the spleen having less than half their own size. While on the move, RBCs have to deal with continuous changes in oxygen tension and pH, have to scavenge reactive oxygen species and need to balance their responses towards the chemical and mechanical challenges.
In contrast, most of the current knowledge about RBCs as well as the diagnostic methods rely on RBCs in relative stasis, such as flux measurements, conventional patch-clamp, calorimetric assays, density centrifugation, atomic force microscopy. In extreme examples, the cells of investigation are even dead like in blood smears, electron microscopy or cyto-spins. Even if cells are on the move like in flow cytometers, they may rest in a drop of liquid. When taken from the circulation, the flow of the RBCs is suddenly absent and (together with the application of anticoagulants) the the RBCs experience a completely different environment that is likely to impair their properties.
The objective of EVIDENCE is the exploration of the properties and behaviour of RBCs under flow conditions and in vivo to understand pathophysiology and to design novel diagnostic devices. Theoretical models will help to understand these RBC properties and will enable the transfer of the gained knowledge into diagnostic devises in general and into the development of a spleen-on-the-chip in particular. Furthermore, we aim to understand the effect of the flow in bioreactors, allowing the efficient production of RBCs in vitro with the goal to produce RBC for transfusion.
The research programme is structured into five work packages (WP1 - WP5) that investigate specific topics: